- 1. Mechanics
- 2. Harmonic Motion, Waves and Sound
- 3. Matter and Thermodynamics
- 4. Electricity and Magnetism
- 5. Light and Optics
- 6. Modern Physics
- 7. Astronomy
- 8. Software and Multimedia
- 9. Index and code conversion from older manual
- External Resources
60. Frank Hertz Experiment
This classic 1914 experiment showed the existence of energy levels and their association with spectral lines. It involves an elaborate setup which requires over an hour of warm-up time and calibration. It is best suited for a lab experiment, or you could arrange to have it set up in a lab, and then bring the students in to watch it. Give plenty of extra notice.
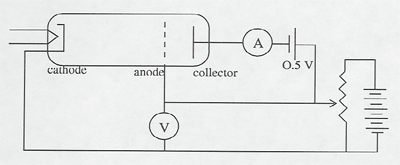
Electrons are accelerated in a nearly evacuated tube with a little mercury vapor in it from the cathode to anode by the voltage V. Those reaching the collector are retarded by 0.5 V. Thus, as V is increased from zero, there is no collector current at A until V > 0.5 V. Then the collector current rises until V reaches the excitation potential of a level in the gas atoms. The inelastic collisions reduce the electrons' energy to zero, and the current drops. As V is increased further, the current again increases until the electrons reach the energy of another level, or double that of the first level. The results can be seen on the current meter as the voltage is increased, or displayed on an oscilloscope screen using a sweep voltage.